Blog Categories
Track Animal Protocol Usage Easily with mLIMS Tools
Tracking protocol usage is essential for maintaining IACUC compliance and managing your animal research efficiently. To simplify this process, mLIMS—developed by BioInfoRx— offers a powerful Protocol Usage feature—helping you stay on top of strain, gene, and pain level quotas with customizable filters and automated counts. Whether you're overseeing one protocol or coordinating multiple studies across teams, the protocol usage tool in mLIMS provides comprehensive oversight, helping you track compliance, prevent overuse, and make informed decisions with confidence.
![]() Flexible Date Range Selection: When you're running monthly reviews or preparing quarterly reports, mLIMS lets you define your reporting window with ease. Fig. 1 shows the Protocol Usage tool in mLIMS, developed by researchers at BioInfoRx. The interface begins with a user-defined time frame for tracking protocol activity.
Flexible Date Range Selection: When you're running monthly reviews or preparing quarterly reports, mLIMS lets you define your reporting window with ease. Fig. 1 shows the Protocol Usage tool in mLIMS, developed by researchers at BioInfoRx. The interface begins with a user-defined time frame for tracking protocol activity.
.png)
Figure 1. Protocol Usage tool in mLIMS.
![]() Clear Status Definitions: One of the key features of the Protocol Usage tool is the ability to define which animal statuses should be excluded from “alive” counts. Options are provided such as dead, sacrificed, demised, missing, moved/exported, in transit or unknown. The animals in those status are excluded from active/live animal counts, ensuring accurate usage summaries and avoiding over counts.
Clear Status Definitions: One of the key features of the Protocol Usage tool is the ability to define which animal statuses should be excluded from “alive” counts. Options are provided such as dead, sacrificed, demised, missing, moved/exported, in transit or unknown. The animals in those status are excluded from active/live animal counts, ensuring accurate usage summaries and avoiding over counts.
![]() Smart Animal Status Counting: Choose how you want the system to treat animals with a Date of Death (DOD) within your selected time frame. Prefer Dead, animals with a DOD within the range will only be counted as dead; Prefer Live, Animals with a DOD within the range will still be considered alive for usage purposes; Double Count, Animals are counted as both live and dead—ideal for overlapping protocols or detailed audits. This flexibility allows research managers and compliance officers to align reporting with internal and regulatory requirements.
Smart Animal Status Counting: Choose how you want the system to treat animals with a Date of Death (DOD) within your selected time frame. Prefer Dead, animals with a DOD within the range will only be counted as dead; Prefer Live, Animals with a DOD within the range will still be considered alive for usage purposes; Double Count, Animals are counted as both live and dead—ideal for overlapping protocols or detailed audits. This flexibility allows research managers and compliance officers to align reporting with internal and regulatory requirements.
![]() Advanced Quota Breakdown: If your protocol includes usage limits by genetic criteria or pain category, mLIMS can display the following quota reports (if available):Protocol Strain Quota Report, Protocol Gene Quota Report, Protocol Pain Level Quota Report. Fig. 2 shows an example report for protocol and strain.
Advanced Quota Breakdown: If your protocol includes usage limits by genetic criteria or pain category, mLIMS can display the following quota reports (if available):Protocol Strain Quota Report, Protocol Gene Quota Report, Protocol Pain Level Quota Report. Fig. 2 shows an example report for protocol and strain.
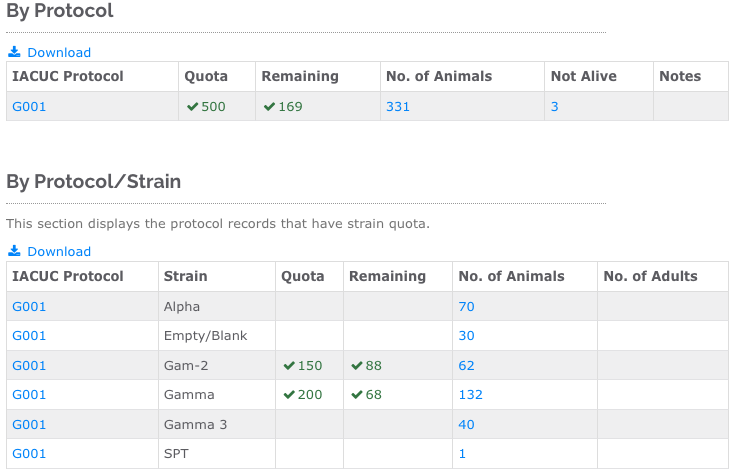
Figure 2. An example report for protocol and strain.
These detailed views ensure that you're staying within your approved limits—no more manual tracking or last-minute panic before protocol renewals. Whether you're managing a single protocol or dozens, this feature is designed to give you clarity and control.